Saving Space on US-Bound Labels
Saving Space on US-Bound Labels
How can you include all the information that you need and want on labels with limited room? Our expert US team shares their top tips for saving label space.
With limited space available on product labels, mandatory and ‘desired’ information (such as communication and branding) continue to battle it out for maximum exposure. Our expert regulatory team shares their top tips for compliantly saving space on labels destined for the US.
What Types of Space-Saving Tactics Are or Are Not Allowed?
- Over Stickers
Over stickers are permitted only if all of the content that is visible at the point of sale is compliant with US FDA ingredients labelling requirements.
- Peel & Reveal
In the US, ‘Peel & Reveal’ is not permitted for the required elements of US food labels, such as the Principal Display Panel (such as the product name and net weight) or Information Panel (such as the Nutrition Facts Panel, ingredient statements, allergens and country of origin).
Mandatory information must be available to read at point of sale without manipulating the label.
But that’s not to say that they can’t be used at all – in fact, some non-mandatory information like recipes and marketing text can utilise the peel & reveal format and provide businesses with additional space to promote their brand or messaging.
- Multinational Labels
It’s not permitted to have another country’s label information on a US panel, according to FDA labelling requirements. The only option to incorporate information from another country would be to utilise dual language labelling. If you do this, note that all content must be presented in US formats (i.e., ingredient statement, allergens, nutrition facts panels).
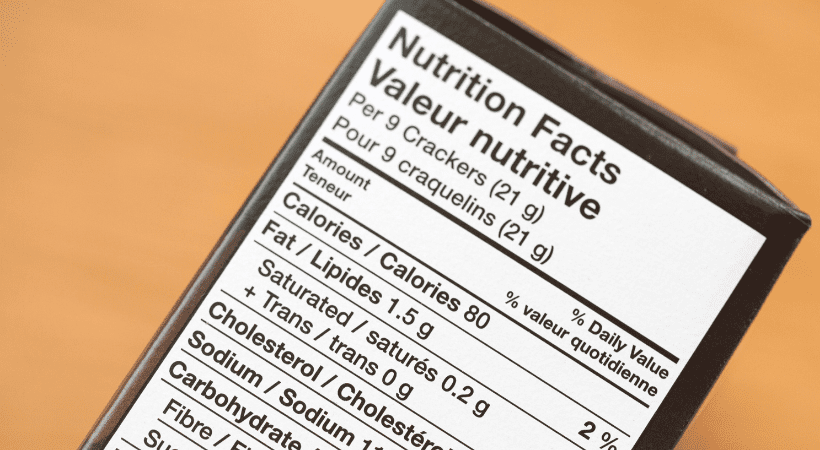
Nutrition Facts Panel
Depending on the size of your label, you can also use the different formats available for the Nutrition Facts Panel, such as:
- Food packages with a surface area <40 sq.in or less for labelling may place the NFP on any label panel (not limited to the information panel); may omit the footnote required if an asterisk is placed at the bottom of the label with the statement “Percent Daily Values are based on a 2,000 calorie diet”; or also use the tabular display label format
- Linear (string) format may be used on food packages with <40 sq.in if the package shape or size cannot accommodate the nutrition information placed in columns on any label panel
- The size of other required elements like the Net Quantity statement can be scaled back too, based on the size of your label
- Small packages <12 sq.in of total surface area available to bear labelling may be printed with a telephone number or an address to obtain nutrition information. This exemption (using a telephone number or address in place of the Nutrition Facts label) is permitted only if there are no nutrient content claims, or other nutrition information on the product label or in labelling and advertising
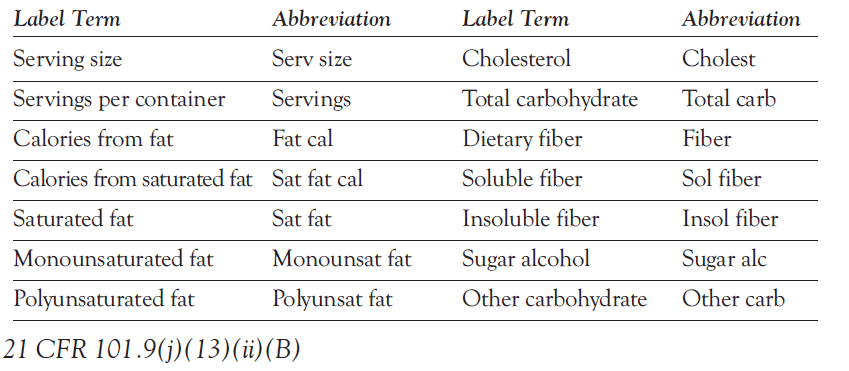
- Food packages with <40 sq.in available for labelling may be able to use abbreviations as found below:

Author: Edward Round
I recently joined Ashbury, bringing with me a decade of experience in marketing across B2B, B2C and SAAS sectors, including finance, insurance and software. My role as Marketing Manager involves managing Ashbury's market positioning and promotional strategies. I work closely with the Client Development and Sales Teams on tasks ranging from targeted outreach programs to creating brand collateral. I thrive on the blend of creativity and analytics that marketing offers, using both to drive successful campaigns and data-driven decisions.
Next reads
The Peanut Diaries: School and Social Occasions
The Peanut Diaries: Navigating Social Events and Celebrations with Food Allergies
The Peanut Diaries: A Parent’s Journey to Uncovering their Child’s Allergy
Redefining Healthy: What the FDA’s New Rules Mean for Food Labels and Nutrition Claims
Keep up to date with our latest insights
Subscribe to our mailing list to stay in touch with the latest news, insights and updates from Ashbury